962
Views & Citations10
Likes & Shares
Background: Accurate estimates
of expected survival times, survival rates of patients and variables that
influence survival of Acquired Immune Deficiency Syndrome are important for
planning health service interventions. The objective of this study was to
estimate mortality rate and to identify survival predictors of adult HIV
infected patients under ART at Wachamo University Nigist Ellen Mohammed
memorial referral hospital.
Methods: A hospital based
retrospective record review was conducted from April to May 2018. All adult HIV
positive patients on antiretroviral treatment at WUNEMM Hospital between
2013and 2017 were included. Data were entered using Epi-Data version 3.1 and
exported to SPS version 20 for analysis. Mortality rate/100 person-years were
calculated. Cox proportional hazards regression was used to predict the risk of
death. Kaplan-Meier curves were also used to estimate survival probability of
HIV infected patients under ART. P-value <0.05 was considered as statically
significant.
Results: The study participants
contributed 1169.39 person-years of observation. Over the study period, the
total mortality rate was 2.22 per 100 person-years at risk. Kaplan-Meier
survival estimation showed that overall estimated survival duration after ART
initiation was 59.85 (95%CI: 58.613-61.091) months. Being on bedridden (AHR:
4.571, 95% CI: (1.344, 15.553) and ambulatory (AHR: 4.028, 95% CI: (1.685,
9.628)) functional status, Co-trimoxazole preventive therapy (AHR: 3.038, 95%
CI: (1.010, 9.145)) and being anemic (AHR: 2.719, 95% CI: (1.060, 6.979)) were
important predictors of mortality.
Conclusion: A lower level of
mortality was detected among adult patients on antiretroviral treatment in
WUNEMMRH. Ambulatory and bed ridden functional status, Co-trimoxazole
preventive therapy and low baseline hemoglobin level were significant
predictors of survival for patients under ART. Strengthening screening program
for early initiation of ART and rising awareness on early treatment seeking
should get due attention to increase the survival of patients on ART.
Keywords: Survival, ART,
HIV/AIDS, Predictors
Abbreviations: AIDS: Acquired Immune Deficiency Syndrome;
ART: Antiretroviral Treatment; BMI: Body Mass Index; CI: Confidence Interval;
CTP: Co-Trimoxazole Therapy; EDHS: Ethiopian Demographic and Health Survey; Hgh:
Hemoglobin; HIV: Human Immune Deficiency Virus; SPSS: Statistical Package for
Social Sciences; PYO: Person Year Observation; UNAIDS: United Nations Programme
on HIV/AIDS; WUNEMMRH: Wachamo University Nigist Eleni Mohamed Memorial Referral
Hospital; WHO: World Health Organization
INTRODUCTION
The Human Immunodeficiency Virus (HIV) [1] causes Acquired Immune
Deficiency Syndrome (AIDS). The vast majority of people living with HIV are
located in low- and middle- income countries, with an estimated 25.5 million
living in sub Saharan Africa. Among this group 19.4 million are living in East
and Southern Africa, which saw 44% of new HIV infections globally in 2016 [2].
ART (Antiretroviral Treatment) began in 2003. Free ART was launched in Ethiopia
in 2005. An estimated 738,976 Ethiopians are currently living with HIV and all
of them require ART [3]. Currently in Ethiopia, 367000 adults and 23400
children are taking ART. Based on 2014 estimate, ART need was 542 121 for
adults and 178500 for children under 15 years of age [4]. Survival patterns
after HIV infection among African populations in the era before ART form a
clear demarcation for measuring future successes of treatment programs [5].
Assessment of the survival patterns among HIV-infected patients who are on ART
is important to determine the effectiveness of the ART program. Moreover,
identifying significant determinants of survival in HIV-infected patients is
necessary to target those at increased risk of death. Mortality rates and its
determinants among HIV patients on ART in different studies across the globe
demonstrate that important determinants differ from place to place. There are
also regional variations of clinical benefit of ART for AIDS patients in terms
of mortality reduction and improved quality of life, with higher rates of case
fatality in poor countries. Even though there have been improvements in service
delivery and utilization, since the introduction of ART services in Ethiopia, a
research not yet available in the study area. Therefore, this study estimated
mortality rate and identified survival predictors of adult HIV infected
patients under ART.
METHODS
Study area
The study was conducted in Wachamo University Nigist Eleni Mohamed
Memorial referral Hospital (WUNEMRH). The Hospital is found in Hosanna Town.
Hossana town is located 230 and 194 km from the capital city of Ethiopia, Addis
Ababa and regional capital city, Hawassa, respectively. The hospital renders
comprehensives HIV/AIDS related services including voluntary counseling and
testing, provider initiated testing and counseling, prevention of
mother-to-child transmission and ART program. During the study, there are about
1103 HIV/AIDS adult patients attending ART in the hospital.
Study design and period
Retrospective cohort study was conducted from
May to June 2018.
Study participants
The study population were all adult (>15
years) HIV positive patients on antiretroviral treatment at WUNEMM Hospital
between 2013 and 2017. Adults (>15 years) HIV-infected patients who had no
follow-up visits and in addition, patients without date of ART initiation and
date of occurrence of events (i.e., death, loss to follow up, and transferred
out) were excluded from study.
Sample size
Total sampling method was used; where all
adult HIV/AIDS patients attending ART in the hospital from January 1, 2013 to
December 30, 2017 was considered for analysis. About 507 records of patients
were included in the study.
Data collection
Data were collected by checklist. Five data
collectors and two supervisors were recruited for data collection. Patients’
chart numbers were collected from ART registration book and by using the chart
numbers, patients’ chart were retrieved by card room workers. Data collected on
demographic factors included age, sex, educational level, marital status,
religion. On clinical characteristics Body Mass Index (BMI), baseline
hemoglobin (Hgh) level, active Tuberculosis (TB) during ART, World Health
Organization (WHO) clinical stages, CD4 count, ART regimen, drug allergy, ART
regimen change and functional status were collected.
DATA ANALYSIS
Data were entered using Epi-Data version 3.1
and exported to SPSS version 20 and STATA version 11 for analysis. Kaplan-Meier
models were used to estimate survival probability after ART initiation. Log
rank tests were used to compare survival curves among the categories of each
variable. Model diagnostics was done using the maximum likelihood estimation
and the Hosmer-Lemeshow goodness-of-fit. The cox-proportional hazard model was
used to assess the relationship between the independent variables and
mortality. The univariate cox-regression analysis was used to estimate the
unadjusted Hazard Ratios (HRs) and the stepwise (backward LR) multivariate cox-regression
analysis was performed to estimate the adjusted hazard ratios. The probability
for the stepwise regression was 0.05 for entry of the variables and 0.10 for
removal of the variables. P-values less than 0.05 were considered as statically
significant.
RESULTS
Socio-demographic characteristics of the participants
About 507 HIV infected adult patients were
included in this study. Of these, 312 (61.5%) were females. The median age was
30 years with inter quartile range (IQR) of 25 to 38 years. About half (50.1%)
of the patients were protestants. Majority (71.8%) patients were married.
Regarding educational level, 199 (39.3%) attended primary education. The mean
BMI was 19.60 (SD=3.02). Half of participants (49.9%) lived in Urban (Table
1).
Clinical characteristics
The baseline mean Hgh of the participants was
13.30 (SD=+2.21). About 69 (13.6%) patients had active TB during treatment. The
mean weight at base line was 52.8 kg (SD=9.65). The baseline median CD4 count
was 239 (cells/μl) (IQR=116-388 cells/dl). About 187 (36.9%) patients were at
WHO clinical stage I and 133 (26.1%) were at WHO clinical stage III. More than
three forth (76.5%) of the participants were in working functional status and 95
(18.7%) were in ambulatory status. Most of adult HIV-infected patients (92.5%)
were recommended TDF+3TC+EF regimen at initiation of treatment. Baseline
regimen change was recommended for 9 (1.8%) patients. Reasons for regimen
change was nausea 6 (1.2%), numbness/tingling/pain 2 (0.4%) and abdominal pain
1 (0.2%). More than half (56.2%) patients’ have used CTP (co-trimoxazole
therapy) (Table 2).
Survival
analysis
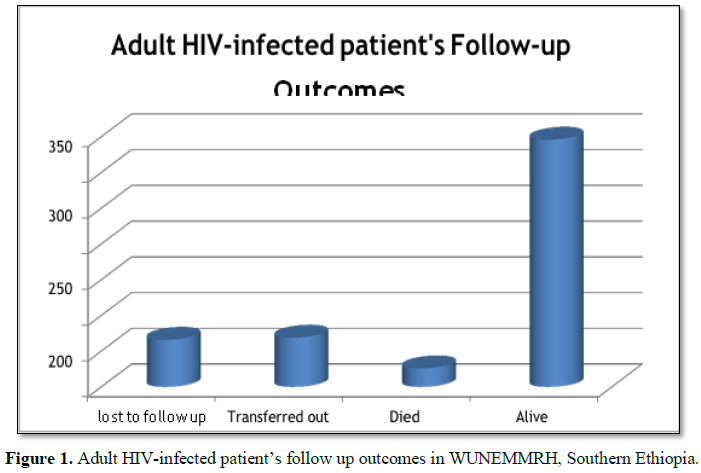
Of 507 HIV infected patients, 66 (13.0%) were
lost to follow up (LTF), 69 (13.6%) transferred out to other ART centers, 26 (5.1%)
died and 346 (68.2%) alive at the 30th December, 2017. Of died
patients, 2 (7.7%) died within 3 months after start of treatment (Figure 1).
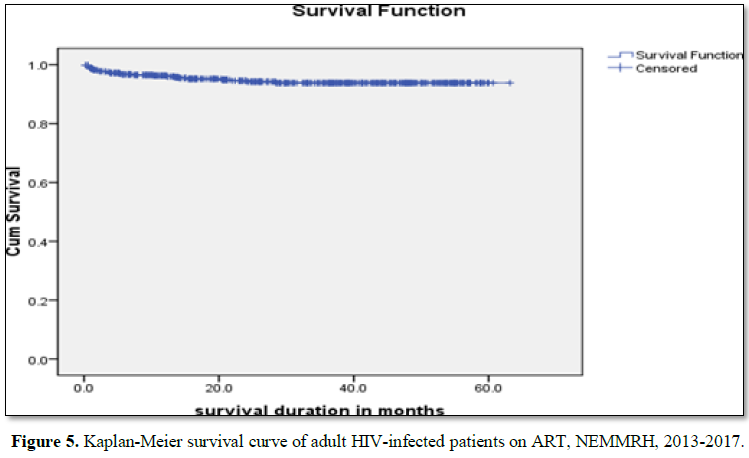
The median survival duration was 26.50 with
an IQR of 11.97 to 43.67 months. Kaplan-Meier survival estimation showed that
overall estimated survival duration after ART initiation was 59.85 months. The
study participants contributed 1169.39 PYO. The total mortality rate was 2.22
per 100 person-years at risk. The rate of LTF was 5.64 per 100 PYO.
Kaplan-Meier analysis of survival status revealed that males show better
survival than females. The estimated survival was 59.235 months in males and in
females. Age 15-29 years indicated higher survival time (Table 3). From
baseline clinical characteristics of patients, those in WHO clinical stage II
survived better, 61.633 months than those in WHO clinical stage IV, 49.409
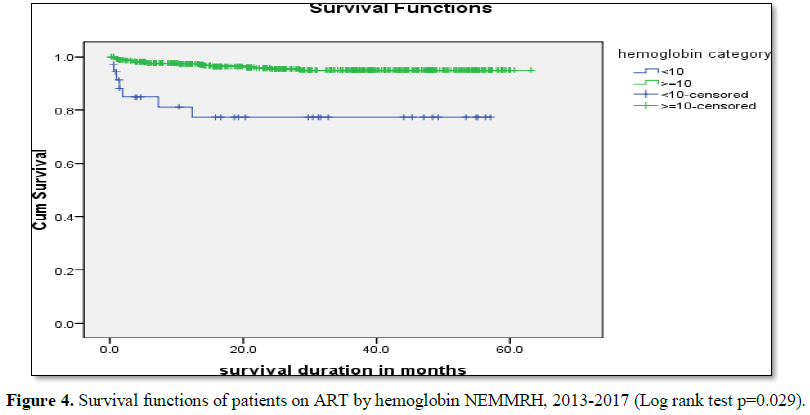
months. Patients with Hgb <10 g/dl showed lesser survival, 45.046 months
than those with higher >10 g/dl Hgb, 60.562 months (Table 4).
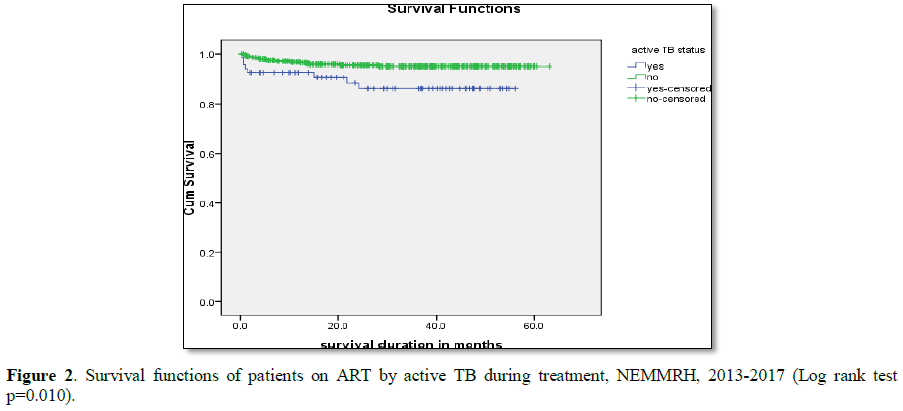
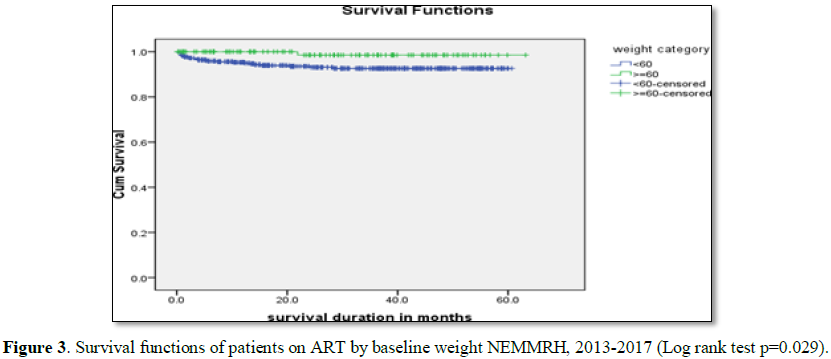
Log rank test for different groups of patients showed differences in
survival and hazard curves. Active TB during treatment (P=0.010), baseline
weight (P=0.029) and Hgh (P<0.001) showed significance association in Log
rank test (Figures 2-5).
The proportional hazards cox regression analysis results
Variables that were significantly associated
at bivariate analysis were further examined in multivariate analysis. Results
of bivariate analysis showed that active TB during treatment (p<0.05),
functional status (p<0.001), WHO stages (p<0.005), baseline CD4+
(p<0.001), baseline Hgb (P<0.001), BMI (p<0.005) and CPT (p<0.005)
as candidate for multivariate analysis (Table 5).
In multivariate proportional hazards cox regression analysis; Functional
status, Baseline Hgh level and CPT were significant predictors of mortality (Table
6). The risk of death was 4 times higher among patients with baseline
ambulatory functional status (AHR: 4.028, 95% CI: (1.685, 9.628) and patients
who with bedridden functional status were about 5 times more likely to die (AHR:
4.571, 95% CI: (1.344, 15.553) compared to patients with working functional
status. Patients who had given CPT were 3 times more likely to die (AHR: 3.038,
95% CI: (1.010, 9.145) compared to who had not given CPT. The hazard of death
was 3 times higher on HIV patients with baseline Hgh level <10 g/dl (AHR:
2.719, 95% CI: (1.060, 6.979)) as compared to those patients with baseline Hgh
level >10 g/dl.
DISCUSSION
AND CONCLUSION
This study tried to estimate mortality rate
and predictors of survival in HIV infected patients under ART at WUNEMMRH. The
overall estimated survival duration after ART initiation was 59.85 months. This
is comparable with study conducted in Debre Markos and Adis Ababa, Ethiopia,
which showed average survival of patients after ART 65.22 and 67 months
respectively [6,7]. In contrary to this study, survival of patients after ART
initiation was 48 months in study conducted in Wollo, Ethiopia. This might be
due to short study period of time, in which death is very high in early periods
of treatment and more than 1000 subjects were in WHO stage IV as compared to
current study [8]. There is also survival difference between current study and
study conducted at Armed Forces Hospital, Ethiopia, which showed survival of
patients after ART initiation 72 months [9]. This difference might be due to
difference in having better information on HIV/AIDS, better clinical care and
nutrition. In current study, the total mortality rate was 2.22 per 100 PYO.
This is similar with study conducted in Adama, which showed mortality rate of
2.1 per 100 PYO. Similarly, study conducted in Ethiopia showed 2.03 per 100
persons at risk [10,11]. However, the finding is in contrary to study conducted
in Nepal, which revealed overall mortality rate of 6.33 per 100 person years at
risk. This high mortality in Nepal might be due to lack of prior access to ART
service, stigma and discrimination related to HIV/AIDS. The difference might
also be due to shortage of diagnostic facility and proper screening of
opportunistic infections and limited availability of prophylaxis. The rate of
LTF was 5.64/100 PYO. This finding is lower than the study of Arba Minch, which
showed LTF rate 8.2/100 PYO. Finding of Adama, Ethiopia, also showed 11.5/100
PYO [11,12]. The median survival time for the study cohort was 26.50 months.
Study conducted in Arba Minch revealed that 13.4% patients were lost from
follow up [11]. Other study conducted in Côte d'Ivoire also showed rate of LTF
program, to a certain extent, reflects the degree of underestimation of
mortality [13]. The risk of death was higher among patients with baseline
ambulatory and bedridden functional status than working functional status. The
finding is in line with study conducted at Armed Forces General Teaching
Hospital [8]. Other studies also showed that ambulatory and bedridden patients
were more likely to die compared to those engaged in active working [10,11,14].
CPT is a feasible, well-tolerated and inexpensive intervention for people
living with HIV to reduce HIV-related morbidity and mortality. However, this
study revealed that patients who had given CPT were more likely to die as
compared to who had not given CPT. The reasons for this might be co-trimoxazole
prophylaxis is recommended for adults (including pregnant women with severe or
advanced HIV clinical disease (WHO stage 3 or 4) and/or with a CD4 count of ≤ 350
cells/mm3. This stage is stage where there is high mortality. In
current study, this high mortality in those who were taking CPT might be
implication for late initiation of ART [15]. The hazard of death was higher on
HIV patients with baseline Hgh level <10 g/dl as compared to those patients with
baseline Hgh level >10 g/dl. This finding was similar with studies of
Ethiopia, which showed patients with anemia were at high risk of death after
ART initiation [10,16-18]. In current study, mortality rate is low. The
estimated mean survival time of patients was 59.85 months. Ambulatory and bed
ridden functional status, CPT and low baseline Hgh level were predictors of survival
for patients under ART. Strengthening screening program for early initiation of
ART and rising awareness on early treatment seeking should get due attention to
increase the survival of patients on ART.
LIMITATIONS
Since secondary data were used, in which some
important variables were not documented well and not analyzed due to missing
values. These are occupation, regimen change and side effects.
DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical clearance was obtained from Ethics
Review Committee of Hossana College of Health Sciences. Permission letter was
obtained from Wachamo University Nigest Elleni Mohamad Memorial Referral
Hospital. Other concerned bodies of the hospital were informed about the study.
All the information retrieved was kept in the way that could not interfere in
personal confidentiality.
AVAILABILITY OF DATA AND MATERIALS
The authors do not have full mandate to share
the data since they are the property of the funding institution.
FUNDING
The study was fully funded by Hosanna College
of Health Sciences. The college also provided experts for supervisory, advisory
and facilitation activities during the study period.
ACKNOWLEDGEMENT
We would like to forward our great thanks to
Hossana College of Health Science for giving us this golden opportunity to
conduct this research. We would also like to thank Wachamo University Nigist
Elleni Mohammed Memorial Referral Hospital for providing relevant information
for this research activity.
COMPETING INTERESTS
The authors declare that they have no
competing interests.
AUTHORS’ CONTRIBUTIONS
TY conceived the research
idea, conducted the data collection, data analysis and data interpretation and
wrote and reviewed the paper. SM conducted the data collection, data analysis
and data interpretation and reviewed the paper. TL approved the
proposal, participated in data analysis and revised subsequent draft of the
paper. All authors read and approved the final manuscript.
1. UNAIDS (2000) AIDS and HIV infection
information for United Nations employees and their families. Geneva.
Switzerland.
2. UNAIDS (2017) Ending AIDS: Progress towards
the 90-90-90 targets. Geneva. Switzerland.
3. Ethiopian Ministry of Health (2017) National
guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa.
4. Ethiopian Public Health Institute (2017) HIV
related estimates and projections for Ethiopia. Addis Ababa.
5. Isingo R, Zaba B, Marston M, Ndege M, Mngara
J, et al. (2007) Survival after HIV infection in the pre-anti-retroviral
therapy era in a rural Tanzanian cohort.
AIDS 21: S5-S13.
6. Assefa T, Wencheko E (2012) Survival analysis
of patients under chronic HIV-care and antiretroviral treatment at Tikur Anbessa
Specialized Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev 26: 22-29.
7. Abebe N, Alemu K, Asfaw T, Abajobir AA (2014)
Survival status of HIV positive adults on antiretroviral treatment in Debre Markos
Referral Hospital, north-west Ethiopia: Retrospective cohort study. Pan Afr Med
J 17: 88.
8. Ayalew J, Moges H, Sahu O, Worku A (2014)
Identifying factors related to the survival of AIDS patients under the
follow-up of antiretroviral therapy (ART): The case of South Wollo. Int J Data
Envelopment Anal Operations Res 1: 21-27.
9. Kebebew K, Wencheko E (2012) Survival
analysis of HIV-infected patients under antiretroviral treatment at the Armed Forces
General Teaching Hospital, Addis Ababa, Ethiopia. Ethiop J Health Dev 26.
10. Biadgilign S, Reda A, Digaffe T (2012)
Predictors of mortality among HIV infected patient taking antiretroviral
treatment in Ethiopia: A retrospective cohort study. AIDS Res Ther 9: 15.
11. Abebe TW, Chaka TE, Misgana GM, Adlo AM (2016)
Determinants of survival among adults on antiretroviral therapy in Adama
Hospital Medical College, Oromia Regional state, Ethiopia. J HIV AIDS 2.
12. Mulissa Z, Jerene D, Lindtjørn B (2010) Patients
present earlier and survival has improved, but pre-ART attrition is high in a
six year HIV cohort data from Ethiopia. PLoS One 5: e13268.
13. Toure S, Kouadio B, Seyler C, Traore M,
Dakoury-Dogbo N, et al. (2008) Rapid scaling-up of antiretroviral therapy in 10,000
adults in Côte d'Ivoire: 2 year outcomes and determinants. AIDS 22: 873-882.
14. Bhatta L (2012) Survival pattern and determinants
of survival in adult HIV-infected patients on antiretroviral treatment in
far-western development region, Nepal. University of Tromsø, Tromsø, Norway
(thesis).
15. WHO (2014) Supplementary section to the 2013
WHO consolidated guidelines on the use of antiretroviral drugs for treating and
preventing HIV infection. Chapter 8 – Prevention, screening and management of
common co-infections.
16. Worku A, San Sebastian M (2009) Pattern and
determinants of survival in adult HIV patients on antiretroviral therapy,
Jimma, Ethiopia (Master thesis).
17. Ergete D (2011) predictors of mortality among
human immunodeficiency virus infected patients' records in Gondar University
Hospital, Ethiopia. University of South Africa (Thesis).
18. Tarekegn S (2011) The effect of HAART on
incidence of tuberculosis among HIV infected patients in Hawassa University
Referral Hospital, South Ethiopia: A retrospective cohort study (Master thesis
in AAU). Addis Ababa University, Ethiopia.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Diabetes (ISSN: 2644-3031)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Pathology and Toxicology Research